Video
Package leaflet
Package leaflet: Information for the user
Terrosa 20 micrograms/80 microliters solution for injection in pre-filled pen
teriparatide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What Terrosa is and what it is used for
- What you need to know before you use Terrosa
- How to use Terrosa
- Possible side effects
- How to store Terrosa
- Contents of the pack and other information
1. What Terrosa is and what it is used for
Terrosa contains the active substance teriparatide that is used to make the bones stronger, and to reduce the risk of fractures by stimulating bone formation.
Terrosa is used to treat osteoporosis in adults. Osteoporosis is a disease that causes your bones to become thin and fragile. This disease is especially common in women after the menopause, but it can also occur in men. Osteoporosis is also common in patients receiving medicines called corticosteroids.
2. What you need to know before you use Terrosa
Do not use Terrosa
- if you are allergic to teriparatide or any of the other ingredients of this medicine (listed in section 6).
- if you have high levels of calcium in your blood (pre-existing hypercalcaemia).
- if you suffer from serious kidney problems.
- if you have ever had bone cancer or if other cancers have spread (metastasised) to your bones.
- if you have certain bone diseases. If you have a bone disease, tell your doctor.
- if you have unexplained high levels of alkaline phosphatase in your blood, which means you might have Paget’s disease of bone (disease with abnormal bone changes). If you are not sure, ask your doctor.
- if you have had radiation therapy involving your bones.
- if you are pregnant or breast-feeding.
Warnings and precautions
Terrosa may increase calcium in your blood or urine.
Talk to your doctor before or while using Terrosa:
- if you have continuing nausea, vomiting, constipation, low energy, or muscle weakness. These may be signs there is too much calcium in your blood.
- if you suffer from kidney stones or have had kidney stones.
Some patients get dizzy or get a fast heartbeat after the first few doses of Terrosa. For the first doses, inject Terrosa in a place where you can sit or lie down right away if you get dizzy.
The recommended treatment time of 24 months should not be exceeded.
Before you start using a new pre-filled pen, write down the batch (Lot) number of the medicinal product and its first injection date on the outer carton of the pre-filled pen and a calendar and provide this information when reporting any side effects.
Terrosa should not be used in growing adults.
Children and adolescents
Terrosa should not be used in children and adolescents (aged less than 18 years).
Other medicines and Terrosa
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
This is important, because some medicines (e.g. digoxin/digitalis, a medicine used to treat heart disease) may interact with teriparatide.
Pregnancy and breast-feeding
Do not use Terrosa if you are pregnant or breast-feeding. If you are a woman of child-bearing potential, you should use effective methods of contraception during use of Terrosa. If you become pregnant while using Terrosa, Terrosa should be discontinued. Ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Some patients may feel dizzy after injecting Terrosa. If you feel dizzy you should not drive or use machines until you feel better.
Terrosa contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per dose, that is to say essentially “sodium-free”.
3. How to use Terrosa
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is 20 micrograms (corresponding to 80 microliters) given once a day by injection under the skin (subcutaneous injection) in the thigh or abdomen.
To help you remember to use your medicine, inject it at about the same time each day. Terrosa can be injected at meal-times.
Inject Terrosa each day for as long as your doctor prescribes it for you. The total duration of treatment with Terrosa should not exceed 24 months. You should not receive more than one treatment course of 24 months over your lifetime.
Your doctor may advise you to use Terrosa with calcium and vitamin D. Your doctor will tell you how much you should take each day.
Terrosa can be given with or without food.
The compatible injection needles are not included with Terrosa.
The pre-filled pen can be used with injection needles developed according to the pen needle ISO standard of a gauge between 29 G and 31 G (diameter 0.25 – 0.33 mm) and a length between 5 mm to 12.7 mm for subcutaneous injection only.
For the correct use of this medicine it is very important to closely follow the detailed Instructions for Use of your pre-filled pen which are provided with the medicine.
Use a new injection needle for each injection to prevent contamination and safely dispose of the needle after use.
Never store your pre-filled pen with the needle attached.
Do not transfer the medicine into a syringe.
You should inject Terrosa shortly after you take the pre-filled pen out of the refrigerator. Put the pen cap on the pre-filled pen and put back into the refrigerator immediately after you have used it. Store it in refrigerator before and during the whole 28-day treatment period.
Preparing for injection
- To ensure the correct administration of Terrosa always read the Instructions for Use of pre-filled Terrosa Pen, which is included in the carton of the medicine.
- Wash your hands before handling the pre-filled pen.
- Check the expiry date on the label of the pre-filled pen before you start using the medicine. Make sure that there are at least 28 days remaining before its expiry date. Write down the batch (Lot) number and the first injection date of the actual pre-filled pen on a calendar. The date of first injection should also be recorded on the outer carton of Terrosa (see the provided space on the box: {First use:}).
- Attach a new injection needle on the pre-filled pen and set the dose on the display window by turning the dose setting knob.
Injecting Terrosa
- Before you inject Terrosa, clean your skin where you intend to inject (thigh or abdomen) as instructed by your doctor.
- Gently hold a fold of cleansed skin and insert the needle straight into the skin. Press the dose setting knob and hold it pressed for at least six seconds to make sure that you receive the whole dose.
- As soon as you have finished the injection, attach the outer needle protective cap on the pen needle and screw the cap anti-clockwise to remove the pen needle. This will keep the remaining Terrosa sterile and prevent leaking from the pre-filled pen. It will also stop air going back into the pre-filled pen and the needle from clogging.
- Replace the cap on your pre-filled pen.
- Dispose of pen needles safely using a sharps container or as advised by your doctor.
If you use more Terrosa than you should
If, by mistake, you have used more Terrosa than you should, contact your doctor or pharmacist.
The expected effects of overdose include nausea, vomiting, dizziness, and headache.
If you forget to use Terrosa
If you forget an injection or cannot use your medicine at your usual time, inject it as soon as possible on that day. Do not use a double dose to make up for a forgotten dose. Do not take more than one injection in the same day.
If you stop using Terrosa
If you are considering stopping Terrosa treatment, please discuss this with your doctor. Your doctor will advise you and decide how long you should be treated with Terrosa.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects are pain in limb (frequency is very common, may affect more than 1 in 10 people). Other common side effects (affecting up to 1 in 10 people) include feeling sick, headache and dizziness. If you become dizzy (light-headed) after your injection, you should sit or lie down until you feel better. If you do not feel better, you should call a doctor before you continue treatment. Cases of fainting have occurred after teriparatide use.
If you have discomfort around the area of the injection such as redness of the skin, pain, swelling, itching, bruising or minor bleeding (which can occur commonly), this should clear up in a few days or weeks. Otherwise tell your doctor.
Rarely (may affect up to 1 in 1 000 people), some patients may suffer allergic reactions consisting of breathlessness, swelling of the face, rash and chest pain. These reactions usually occur soon after injection. In rare cases, serious and potentially life-threatening allergic reactions including anaphylaxis can occur.
Other side effects include:
Common (may affect up to 1 in 10 people)
- increase in blood cholesterol levels
- depression
- nerve pain in the leg
- feeling faint
- spinning sensation
- irregular heartbeats
- breathlessness
- increased sweating
- muscle cramps
- loss of energy
- tiredness
- chest pain
- low blood pressure
- heartburn (painful or burning sensation just below the breast bone)
- vomiting
- a hernia of the tube that carries food to your stomach (hiatus hernia)
- low haemoglobin or red blood cell count (anaemia).
Uncommon (may affect up to 1 in 100 people)
- increased heart rate
- abnormal heart sound
- shortness of breath
- piles (haemorrhoids)
- leakage of urine
- increased need to pass water
- weight increase
- kidney stones
- pain in the muscles and pain in the joints. Some patients have had severe back cramps or pain which led to admission into hospital.
- increase in blood calcium level
- increase in blood uric acid level
- increase in an enzyme called alkaline phosphatase.
Rare (may affect up to 1 in 1 000 people)
- reduced kidney function, including renal failure
- swelling, mainly in the hands, feet and legs.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Terrosa
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and the pre-filled pen after EXP. The expiry date refers to the last day of that month.
Store in a refrigerator (2 °C – 8 °C) at all times. The pre-filled pen should be returned to the refrigerator immediately after use. Do not freeze.
Keep the pen cap on the pre-filled pen in order to protect from light.
You can use Terrosa for up to 28 days after the first injection, as long as the pre-filled pen is stored in a refrigerator (2 °C to 8 °C).
Avoid placing the pre-filled pen close to the ice compartment of the refrigerator to prevent freezing. Do not use Terrosa if it is, or has been, frozen.
Each pre-filled pen should be properly disposed of after 28 days of first use, even if it is not completely empty.
Terrosa contains a clear and colourless solution. Do not use Terrosa if solid particles appear or if the solution is cloudy or coloured.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Terrosa contains
- The active substance is teriparatide. Each dose of 80 microliters contains 20 micrograms of teriparatide. One pre-filled pen of 2.4 mL contains 600 micrograms of teriparatide (corresponding to 250 micrograms per mL).
- The other ingredients are: glacial acetic acid, mannitol, metacresol, sodium acetate trihydrate, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment), water for injections (see section 2 “Terrosa contains sodium”).
What Terrosa looks like and contents of the pack
Terrosa is a colourless and clear solution for injection (injection). It is supplied in a pre-filled pen. Each pre-filled pen contains 2.4 mL of solution, enough for 28 doses.
Pack sizes: 1 pre-filled pen or 3 pre-filled pens in a carton.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Gedeon Richter Plc.
Gyömrői út 19-21.
1103 Budapest
Hungary
This leaflet was last revised in
Other sources of information
Detailed information on this product is also available by scanning the QR code included below or the outer carton with a smartphone. The same information is also available on the following URL: www.terrosapatient.com

Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu
Pen user manual
Terrosa 20 micrograms (µg)/80 microliters solution for injection, in pre-filled pen
Instructions for Use
Important information
This pre-filled Terrosa Pen is for administering a daily fixed dose of 80 microliter Terrosa solution for injection to treat osteoporosis.
Your pre-filled pen contains 28 doses.
There is no possibility to set a dose other than 80 microliter.
If you cannot set the 80 microliter dose, your pen is nearly empty.
Terrosa Pen is not refillable, do not use after 28 doses.
Guide to parts
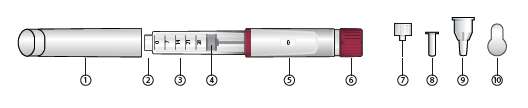
1. Cap
2. Needle connector
3. Cartridge holder
4. Cartridge plunger
5. Display window
6. Dose setting knob
7. Pen needle
8. Inner needle shield
9. Outer needle cap
10. Peel-off seal
Pen needle is not included in the pen packaging. It needs to be attached before using the pen (see step 1c).
Before you start
Before starting to use your new Terrosa pen, please read these instructions all the way through. Follow the directions carefully when using the pen. Also read the package leaflet provided.
Write down your first injection date on a calendar.
Do not share your pen or your needles as this may carry the risk of transmitting infectious agents.
Wash your hands before every injection.
Do not use your Terrosa Pen if it is damaged or if you have any doubts about its correct functioning.
Prepare everything you need:
• alcohol swabs
• the prefilled pen
• a pen needle
1. Preparing your pen
1/a Remove pen cap
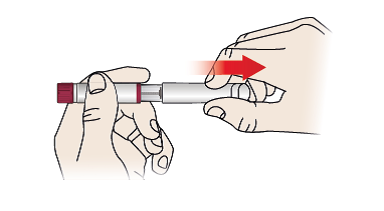
- Remove pen cap (1) by pulling off
1/b Check the medicine
- Check the Terrosa Pen label to make sure you have the correct medicine and that it has not expired.
- Do not use your Terrosa Pen if:
- the pen is damaged.
- the solution is cloudy, coloured or contains particles.
1/c Attach needle
- Take a new pen needle for each injection, and only use pen needles recommended in section “Compatible pen needles” on the back page of this instructions for use.
- Do not use the pen needle if the peel-off seal is damaged or loose – throw it away and take another one.
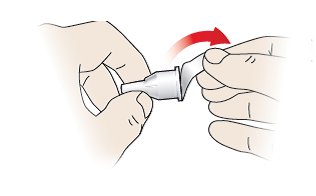
- Remove the peel-off seal (10).
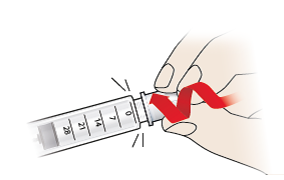
- Press the needle onto the tip of the pen and screw on until firmly attached.
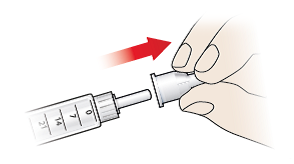
- Pull the outer needle cap (9) away and keep it safe as you will need it to remove the needle later. Keep the inner needle shield (8) on the needle to avoid accidental needle stick injuries.
2. Injection
2/a Set dose
Your pen contains 28 fixed doses of 80 microliters. This fixed dose should be set for every daily injection.
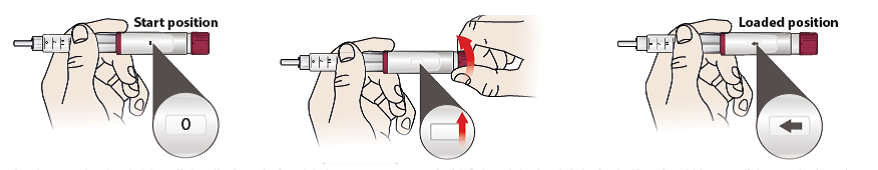
- Turn the dose setting knob (6) until the display window (5) shows an arrow symbol and the knob is locked – You should hear a click sound when the dose is correctly set.
- The arrow sign means that the fixed daily dose is set and the pen is ready for injection.
- If you cannot set the dose the pen is nearly empty. Use another pen.
2/b Choose injection site
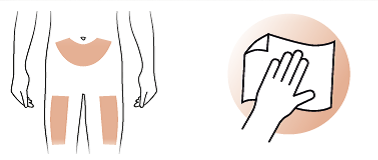
- Use your abdomen or upper thigh for injection. Prepare your skin as recommended by your doctor.
- Clean the chosen area with an alcohol wipe.
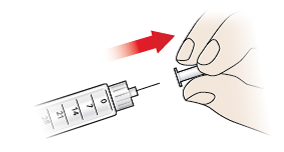
- Carefully pull away the inner needle shield (8) and dispose of it immediately.
2/c Deliver dose
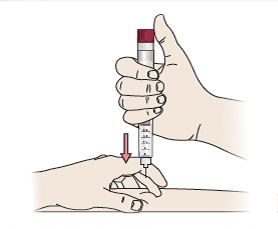
- Gently hold a skin fold between the thumb and index finger. Insert the needle straight and gently into the skin.
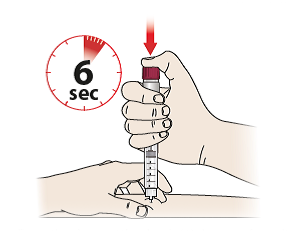
- Press the dose setting knob (6) down as far as it will go and keep it pressed for at least 6 seconds to ensure that the full dose is delivered – You will hear a click sound when start pressing the knob, which is normal.
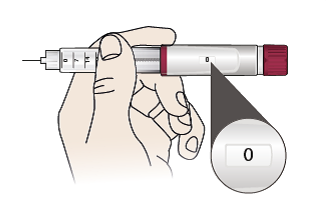
- Pull out the pen slowly. Important: Check if the display shows ‘0’.
3. After injection
3/a Remove needle
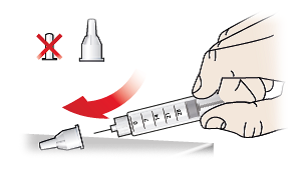
- Carefully push the outer needle cap (9) back onto the pen needle (7).
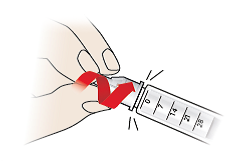
- Grip the outer needle cap (9) and unscrew the pen needle (7).
- Always dispose of pen needles safely using a sharps container or as advised by your doctor.
3/b Storing your pen
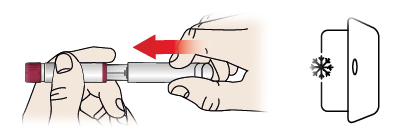
- Put the pen cap (1) back on the pen.
- Put the pen back into the refrigerator between 2 and 8 °C immediately after use.
3/c Pen disposal

When you need to dispose of your pen after 28 days of use, do so carefully, and as described in section “Disposal of Terrosa Pen and used needles” on the back page of this Instructions for use.

Troubleshooting
Follow the instructions given in the table if you have any questions regarding the use of Terrosa Pen:
| Problem | Solution |
| 1. Small air bubbles are visible in the cartridge. | A small air bubble will not affect the dose or cause any harm. |
| 2. Needle cannot be attached. | Use another needle instead. If the second needle cannot be attached either, contact the customer service. |
| 3. Needle is broken/curved/kinked. | Use another needle instead. |
| 4. If accidently tried to inject without a needle attached. | Attach a needle. You will observe some drops coming out. The pen is now again ready for use. Set the dose and inject. |
| 5. The dose setting knob cannot be turned up to the arrow sign. | The amount of medicine left in the Terrosa Pen is less than 80 microliters. Use a new Terrosa Pen. |
| 6. Display does not return to ‘0’ position after injection. | Do not repeat the injection on the same day. Use a new needle and do the injection on the following day. If the display still does not return to ‘0’ position after injection, do not use this pen; contact the customer service. |
| 7. Spillage from pen is observed. | Do not use this pen; contact the customer service. |
Additional important information
- The prefilled Terrosa Pen contains 28 daily doses of fixed 80 microliters Terrosa solution for injection to treat osteoporosis.
- Do not transfer the medicine into a syringe.
- Use your Terrosa Pen only as prescribed by your doctor, in this Instructions for Use and the Terrosa package leaflet.
- Use a new needle for each injection.
- The Terrosa Pen can be used by self-injecting patients above the age of 18 years, healthcare professionals or third parties such as, for instance, adult relatives.
- The Terrosa Pen must not be used by blind or visually impaired patients without help from a trained able-bodied person. Consult your doctor in the case of hearing or handling problems.
If you have any questions concerning the use of the Terrosa Pen, please contact our customer service.
Telephone number: XXXXXXXXXX
Email: XXXXXXXX
Compatible pen needles
- Clickfine pen needle 29 to 31 gauge (diameter 0.25 – 0.33 mm) and 12, 10, 8 or 6 mm length.
- BD Micro-Fine pen needle 29 to 31 gauge (diameter 0.25 – 0.33 mm) and 12.7, 8 or 5 mm length.
Storage and care of Terrosa Pen
- Do not store the Terrosa Pen with a needle attached as this may cause air bubbles to form in the cartridge.
- Transport and store the Terrosa Pen at temperatures between 2 – 8 °C.
- Do not store your Terrosa Pen in the freezer. If the medicine has been frozen, throw the device away and use a new Terrosa Pen.
- Store your Terrosa Pen and pen needles out of reach of children.
- Handle your pen with care. Do not drop your pen and avoid knocking it against hard surfaces. Protect it from water, dust and moisture.
- A damp cloth is sufficient to clean the Terrosa Pen. Do not use alcohol, other solvents or cleaning agents. Never immerse the Terrosa Pen in water, as this could damage the pen.
- Do not use your Terrosa Pen if it is damaged or if you have any doubts about its correct functioning.
Disposal of the Terrosa Pen and used needles
- Dispose of the Terrosa Pen 28 days after first use.
- Before disposing of the Terrosa Pen always remove the pen needle.
- Put used needles in a sharps container or a hard plastic container with a secure lid. Do not throw needles directly into your household waste.
- Do not recycle the filled sharps container.
- Ask your doctor or pharmacist about options to dispose of the pen and the sharps container properly.
- The directions regarding needle handling are not intended to replace local, healthcare professional or institutional policies.
Marketing authorization holder: Gedeon Richter Plc., Hungary
Manufactured by Gedeon Richter Plc., Hungary
This user manual was last revised in


